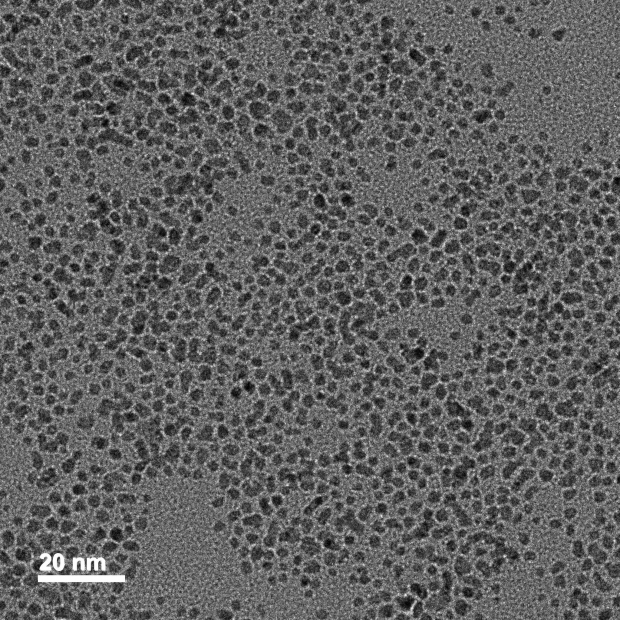
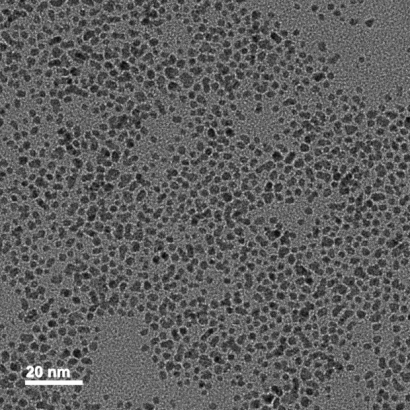
Average size : 3±2 nm
Size distribution ( CV% ) : < 10%
Peak wavelength : 518-520 nm
Platinum Nanoparticle Applications
Platinum nanoparticles are used in several important catalytic applications including fuel cells and photocatalysts. Platinum nanocrystal arrays have also been studied as nonvolatile memory systems.
A platinum colloid is a suspension with a dispersed phase consisting of platinum particles between 1 nm – 1 µm evenly dispersed in a continuous phase, in this case water. The sample can be considered to be on the nanoparticle scale if the size of the dispersed phase particles is between 1-100 nm. Platinum colloidal particles are stabilized by coating their surfaces with high polymers to form 3-D barriers or using the reduction potential of platinum to impose repulsive forces between the particles. Platinum nanoparticles have found applications in catalysts for treating automobile exhaust gas and fuel cell electrodes. Other applications of platinum nanoparticles include biosensors utilizing the optical properties
Citrate Surface
Citrate is one of the most common stabilizing molecules for metal nanoparticles. It provides a highly negatively charged surface that can be displaced by many other molecules or ligands such as those with terminal amines or mercapto groups. Citrate is a small molecule with multiple carboxylic acid groups that weakly associate with the particle surface. It has good stability in water and weakly-buffered solutions. However, citrate doesn’t provide steric stability and noble metal nanoparticles with citrate on their surface are susceptible to aggregation in high salt solutions and non-aqueous solvents.
Advantages :
Highly displaceable surface for performing ligand exchange with proteins or other ligands. Molecules with thiols or amines will readily displace citrate from the surface and strongly associate with gold or silver surfaces.
Small change to hydrodynamic diameter – TEM measured diameter is very close to hydrodynamic diameter as measured with DLS.
Property Highlights
Displaceable: Citrate is more displaceable than tannic acid but less displaceable than carbonate.
Negatively charged
Salt stability: Destabilized in low salt concentrations.
Toxicity: Very low